You are now leaving XTANDI.com
The website you are about to visit is not owned or controlled by Astellas. Astellas are not responsible for the information or services on this site.
- Conduct collaborative research on Sony's unique polymeric materials to achieve high-DAR ADC -
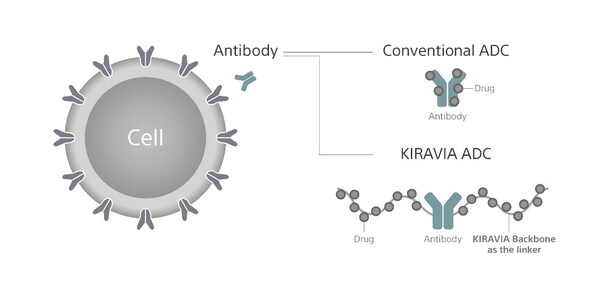
TOKYO, May 16, 2023 /PRNewswire/ -- Sony Corporation (President and CEO: Kimio Maki, "Sony") and Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, "Astellas") today announced that they have entered into a collaborative research agreement to discover a novel Antibody-Drug Conjugate (ADC)*1 platform in oncology based on Sony's unique polymeric material, "KIRAVIA™*2 Backbone*3." ADC is expected to selectively deliver anti-cancer drugs to target cells, thereby increasing efficacy and reducing side effects caused by anti-cancer drugs attacking normal cells. The technology to create linkers which conjugates antibodies and drugs, is considered to be a key to development of a better-performing ADC. This collaborative research leverages the flexibility in design and resulting properties such as high capacity and solubility of KIRAVIA Backbone as a linker of ADC, to effectively deliver anti-cancer drugs to targeted cells in a stable manner, aiming to further enhance therapeutic efficacy by achieving high Drug-to-Antibody Ratio (DAR) etc.
The two companies jointly began exploratory research of new linker technology aimed at creating a new ADC platform in July 2022, and the expected profile was obtained in feasibility studies using human cancer cells. Under this agreement, Sony and Astellas will jointly develop and optimize a new ADC platform using the KIRAVIA Backbone as a linker. In addition, Astellas will conduct non-clinical trials of development candidates. Furthermore, in order to build a drug discovery platform not limited to ADC, the two companies have agreed to continue discussions on expanding research partnerships to create new value by combining Sony's cutting-edge technology with Astellas' renowned pharmaceutical capabilities.
"Sony's life science business has accumulated substantial knowledge in the field of cell analysis," said Katsunori Ogawa, Head of Life Science & Technology Business Unit at Sony Corporation. "Through this collaboration, Sony is striving to contribute to the medical and drug discovery fields and provide further social value by leveraging Sony's technological capabilities in the development of anti-cancer drugs therapy, which are expected to grow."
"We are pleased to enter into a joint research agreement with Sony," said Yoshitsugu Shitaka, Ph.D., Chief Scientific Officer (CScO), Astellas Pharma Inc. "Astellas is working to create innovative drugs from a multifaceted perspective called the Focus Area approach*4, which identifies combinations of biology, therapeutic modality or technology and diseases with high unmet medical needs. The partnership will further strengthen our ability to utilize suitable modalities. It is our expectation that the collaboration will lead to the continuous creation of innovative drugs for patients around the world."
*1: ADC is a modality (Types of therapeutic methods such as low-molecular-weight drugs, antibody drugs, nucleic acid drugs, cell therapy, and gene therapy) that combines an antibody and a small molecule such as an anti-cancer drug via a linker. It is expected to selectively deliver anti-cancer drugs to target cells, thereby reducing side effects caused by anti-cancer drugs attacking normal cells.
*2 KIRAVIA™ and KIRAVIA Dyes™ are registered trademarks or trademarks of Sony Group Inc. or its affiliates.
*3: KIRAVIA Backbone is created using the organic polymer technology cultivated in KIRAVIA Dyes™, which Sony independently developed and licensed to reagent manufacturers. It features a high degree of freedom in design, as the three-dimensional structure is programmed and polymerized using an automatic synthesizer. While increasing the number of drugs to be added (achieving high-DAR), it is highly stable, such as loading multiple types of drugs, improving water solubility (prevention of aggregation), and cleaving by reacting with intracellular enzymes. Synthesis of linkers with functional properties and selective drug release is expected.
*4: Astellas has established a Focus Area Approach for its research and development strategy. Specifically, our Focus Area Strategy is defined as combinations of three components: (1) biology with high disease relevance, (2) versatile modalities/technologies, and (3) diseases with high unmet medical needs that are expected to be tackled by the first two components. We view this strategy as a way to build a sustainable, expandable drug discovery approach to develop new platforms, leverage expertise and create innovative products
About Sony
Sony Corporation is a wholly owned subsidiary of Sony Group Corporation and is responsible for the Entertainment, Technology & Services (ET&S) business. With the vision of "continuing to deliver Kando and Anshin(*) to people and society across the world through the pursuit of technology and new challenges," Sony Corporation supports the Sony Group with technology to create the entertainment of the future together with creators.
For more information, visit: http://www.sony.net/
*Both Japanese words, Kando means emotion and Anshin has various meanings such as peace of mind, reassurance, reliability and trust.
About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into VALUE for patients. For more information, please visit our website at https://www.astellas.com/en.
Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management's current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas' intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
SOURCE Astellas Pharma Inc.
 |
Get only the email alerts you want.
For media inquiries and reporter requests, please email us at corporate.communications@us.astellas.com.
Our communications team will respond to verified media requests within 24-48 hours as appropriate.
If you are not a reporter and need assistance, please visit our contact us page that includes information for patients, healthcare providers and researchers.
This website is intended for U.S. residents only. This website contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, or in different dosages. Nothing contained herein should be considered a solicitation, promotion or advertisement for any drug including those under development. Any information on the products contained herein is not intended to provide medical advice nor should be used as a substitute for the advice provided by your physician or other healthcare provider.
The site uses cookies to provide you with a more responsive and personalized service and to analyze site traffic. By using this site, you accept our use of cookies as described in our privacy policy. Please read our privacy policy for more information on the cookies we use, the processing of your personal data and how to delete or block the use of cookies.