You are now leaving XTANDI.com
The website you are about to visit is not owned or controlled by Astellas. Astellas are not responsible for the information or services on this site.
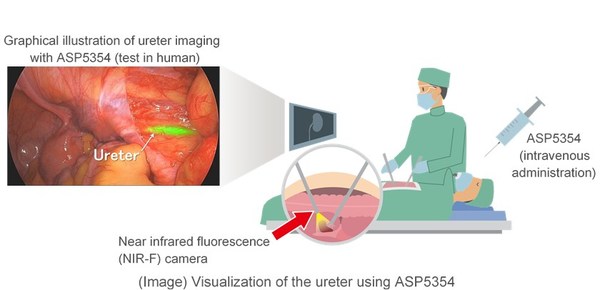
TOKYO, Oct. 28, 2020 /PRNewswire/ -- Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., "Astellas" ) announced today that the United States ("U.S.") Food and Drug Administration (FDA) has granted Fast Track designation based on nonclinical and clinical data for the development of ASP5354, an imaging agent being investigated for intraoperative ureter visualization in patients undergoing minimally invasive and open abdominopelvic surgeries. The FDA's Fast Track Designation Program aims to expedite the development and review of potential treatments for serious or potentially life-threatening illnesses with high unmet medical needs. The ASP5354 Fast Track designation is expected to faciliate development and potentially early clinical availability with faster reviews of the novel agent.
ASP5354 is an optical imaging agent being developed by Astellas' Rx+® business1 as a surgical adjunct to lessen the likelihood of iatrogenic ureteral injury (IUI) during complex abdominal and pelvic surgeries such as colorectal or gynecologic surgeries. IUI can result in long-term complications such as ureteral stricture / obstruction, ureteral-vaginal fistulae, acute or chronic renal failure, or sepsis. A survey of more than 2 million surgical cases in the U.S. shows that IUI is accompanied by higher rates of morbidity and, in some cases, mortality. Additionally, because of the need for potentially extensive reconstructive surgeries and extended hospitalizations, the costs to manage IUI can be exceedingly high2.
ASP5354 is a derivative of indocyanine green (ICG) that fluoresces upon excitation with a particular wavelength of near-infrared light. When administered intravenously, ASP5354 is primarily and rapidly excreted by the kidneys and provides the surgeon with visualization of the ureter(s) during surgery through the use of a near-infrared fluorescence (NIR-F) medical device. ASP5354 is an investigational compound discovered by Mie University and Nagoya University, with Astellas acquiring exclusive development and marketing rights worldwide.
In the Phase 1 study ASP5354 was safe and well tolerated at all doses evaluated in healthy volunteers. A Phase 2 study is currently underway to evaluate the safety and efficacy of ASP5354 in patients undergoing colorectal surgery. For more information on ASP5354 clinical trials, please visit clinicaltrials.gov.
Through its Rx+® business, Astellas aims to realize a society where people can live in their own way, both physically and mentally through scientifically based healthcare solutions. Through this venture, Astellas aims to optimize treatment by improving diagnostic accuracy and surgical outcomes. The development of ASP5354 for precision surgery is part of this effort. Going forward, Astellas looks forward to providing various solutions that contribute to more precise and safer surgical methods through the fusion of medical device innovation and pharmaceutical technology.
(1) Rx+® business: A business that leverages the expertise and knowledge of Astellas, which has been cultivated through its prescription drug (Rx) business, integrates innovative medical technology with cutting-edge technology in different fields, contributes to patients through Patient Journey (overall medical care, including diagnostic, preventive, therapeutic, and prognostic care), and creates new revenue streams separate from Astellas' core Rx products.
For more information, please visit https://www.astellas.com/en/partnering/rx-plus
(2) Halabi WJ et al. Ureteral injuries in colorectal surgery: an analysis of trends, outcomes, and risk factors over a 10-year period in the United States. Dis Colon Rectum. 2014; 57:179-86.
About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into value for patients. For more information, please visit our website at https://www.astellas.com/en.
Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management's current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas' intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
SOURCE Astellas

 |
Get only the email alerts you want.
For media inquiries and reporter requests, please email us at corporate.communications@us.astellas.com.
Our communications team will respond to verified media requests within 24-48 hours as appropriate.
If you are not a reporter and need assistance, please visit our contact us page that includes information for patients, healthcare providers and researchers.
This website is intended for U.S. residents only. This website contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, or in different dosages. Nothing contained herein should be considered a solicitation, promotion or advertisement for any drug including those under development. Any information on the products contained herein is not intended to provide medical advice nor should be used as a substitute for the advice provided by your physician or other healthcare provider.
The site uses cookies to provide you with a more responsive and personalized service and to analyze site traffic. By using this site, you accept our use of cookies as described in our privacy policy. Please read our privacy policy for more information on the cookies we use, the processing of your personal data and how to delete or block the use of cookies.