You are now leaving XTANDI.com
The website you are about to visit is not owned or controlled by Astellas. Astellas are not responsible for the information or services on this site.

A new report released by Astellas captures how current-generation immunosuppressant medications have improved the success rates of solid organ transplantation, saving and extending lives, reducing healthcare costs, and paving the way for future regenerative and cell-based therapies that have the potential to transform patient care.
The report, “Value of Medicine in Solid Organ Transplantation,” addresses the more than half-century history of solid organ transplantation surgeries, observing that they were characterized initially by organ rejections, graft losses, and poor health outcomes. The development of immunosuppressive therapies, combined with improved surgical techniques and regulatory advances, have led to improved life expectancies and enhanced quality of life for transplant patients.

“The timeline of solid organ transplantation medications is critical to the history of modern medicine, but it’s also previewing our healthcare future,” said Shontelle Dodson, senior vice president and head of Astellas Medical Affairs Americas. “Thanks to current immunosuppressant drugs, patients with end-stage organ disease can lead longer, productive lives. Multiple-year survival rates for kidney, heart, liver and other transplants are continually climbing, and patients are experiencing few physical limitations and improved emotional well-being. And what we’re learning from immunosuppressant research is opening the doors to new, cutting-edge medical innovations.”
While transplantation began in the mid-1950s, it was only the development of initial-stage immunosuppressants that began to reduce graft rejection rates and improve the prospects of post-transplantation survival. The Food and Drug Administration’s approval of cyclosporine in 1983 was critical in transforming solid organ transplantation from an experimental option to a viable treatment choice. Today, five-year post-transplant patient survival rates are extremely high – 86% for kidney patients, 80% for pancreas, 78% for heart, 75% for liver. Virtually every patient who receives a kidney transplant today will survive the first year, an outcome once thought unimaginable.
Patient Survival Rates by Type and Years Post-Transplantation
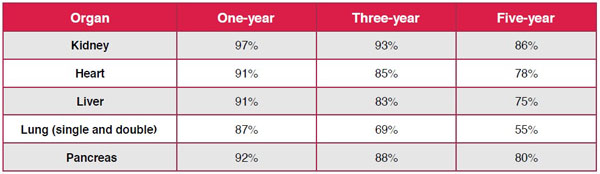
Bentley TS, Phillips SJ. Milliman Research Report. 2017 U.S. organ and tissue transplant cost estimates and discussion. http://www.milliman.com/uploadedFiles/insight/2017/2017-Transplant-Report.pdf. Accessed October 13, 2017.
This continuing progress will have a beneficial impact on healthcare costs. The cost per patient for each year on kidney dialysis is approximately $121,000. The average Medicare reimbursement for a patient in the first post-transplant year is an average $83,401, with annual costs in successive years averaging just over $25,000. So, over the course of a normal lifetime for a patient with end-stage kidney disease, cost savings from transplantation in place of dialysis will be as much as $735,000.
These cost figures make a larger, important point about the relationship between innovation and health system sustainability. Without the innovation that has taken place in immunosuppressant medications, patients would still be reliant upon a long-term, high-cost dialysis treatment regimen that leaves them relegated to a diminished quality of life. Improved patient health yields reduced health system costs.
The report, which was authored by Xcenda L.L.C. on behalf of Astellas, closes by observing, “While the development of novel immunosuppressants have improved patient outcomes, research in this field is still ongoing. Immunosuppressants have opened doors for newer cutting-edge approaches to treat end-stage organ disease and paved the way for future research involving regenerative medicine and cell-based therapies that can further improve patient lives.”
Click here to access the full report, including related citations.
 |
Get only the email alerts you want.
For media inquiries and reporter requests, please email us at corporate.communications@us.astellas.com.
Our communications team will respond to verified media requests within 24-48 hours as appropriate.
If you are not a reporter and need assistance, please visit our contact us page that includes information for patients, healthcare providers and researchers.
This website is intended for U.S. residents only. This website contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, or in different dosages. Nothing contained herein should be considered a solicitation, promotion or advertisement for any drug including those under development. Any information on the products contained herein is not intended to provide medical advice nor should be used as a substitute for the advice provided by your physician or other healthcare provider.
The site uses cookies to provide you with a more responsive and personalized service and to analyze site traffic. By using this site, you accept our use of cookies as described in our privacy policy. Please read our privacy policy for more information on the cookies we use, the processing of your personal data and how to delete or block the use of cookies.